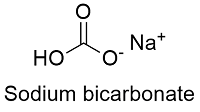
Sodium hydrogen carbonate is also known as sodium bicarbonate and baking soda. Its chemical formula is
Preparation of sodium hydrogen carbonate:
It is obtained as an intermediate product in Solvay’s process for the manufacture of sodium carbonate. In the Solvay’s process, carbon dioxide, water, ammonia, and brine solution in its concentrated form are used as raw materials. The process is widely used because it is inexpensive and less number of raw materials are used to produce the desired chemical. The reaction proceeds as described here: $NH_{3}+ Na^{+}+ CO_{2}+ H_{2}O \to NaHCO_{3}+ NH_{4}^{+}$
Or it can be shown in two steps as:
- The Reaction of aqueous sodium hydroxide and carbon dioxide:$NaOH+ CO_{2} \to Na_{2}CO_{3}+ H_{2}O$
- Addition of more amount of carbon dioxide in step-1 reaction: $Na_{2}CO_{3}+ CO_{2}+ + H_{2}O \to 2NaHCO_{3}$
Sodium hydrogen carbonate can also be prepared by saturating a solution of sodium carbonate with carbon dioxide. Sodium hydrogen carbonate being less soluble separates out as white crystals. The reaction is shown below:
$Na_{2}CO_{3}+ H_{2}O+CO_{2} \to 2NaHCO_{3}$
Properties of sodium hydrogen carbonate:
- Sodium bicarbonate is a white crystalline solid.
- Sodium hydrogen carbonate is non-flammable.
- The melting point of sodium hydrogen carbonate is 323 K.
- Sodium bicarbonate is basic in nature.
- The density of sodium bicarbonate is 2.20 g/mL.
- It is only sparingly soluble in water and poorly soluble in acetone and methanol. However, it is insoluble in ethanol.
- Sodium bicarbonate consists of two ions sodium$(Na^{+}) $ and bicarbonate ($HCO_{3}^{-})$. It dissolves into the water by giving two ions as shown here: $NaHCO_{3} \to Na_{(aq)}^{+ }+ HCO_{3}^{-}$
- Sodium bicarbonate upon heating to 373 K, it decomposes to form sodium carbonate as shown here: $2NaHCO_{3} \to Na_{2}CO_{3}+ H_{2}O+CO_{2}$
- Its aqueous solution is alkaline in nature. $NaHCO_{3}+ H_{2}O \to NaOH+ H_{2}O+CO_{2}$
- Sodium bicarbonate is an amphoteric compound, which means that the compound has both acidic as well as alkaline character. The reaction with acids results in sodium salts and carbonic acid and reaction with basic solution produce carbonates and water. The reactions are shown here:
$NaHCO_{3}+ HCl \to NaCl+ H_{2}CO_{3}$$NaHCO_{3}+ NaOH \to Na_{2}CO_{3}+ H_{2}O$
Uses of sodium bicarbonate:
- It is mainly used in making baking powder. Baking powder contains sodium hydrogen carbonate and an acid like tartaric acid or citric acid. When baking powder is heated sodium hydrogen carbonate decomposes to give carbon dioxide and sodium carbonate. Carbon dioxide bubbles out causing the bread and cakes to rise leaving holes in cakes or pastries. This makes them light and fluffy. Tartaric acid or citric acid present in the baking powder reacts with sodium bicarbonate and neutralizes it.
- Baking soda is used in medicines as an antacid to remove the acidity of the stomach. Sodium bicarbonate is a mild antiseptic for skin infections.
- It is used in fire extinguishers. Soda acid fire extinguisher contains a solution of sodium hydrogen carbonate and sulphuric acid. These can be brought in contact with each other either by pressing a knob or by inverting the extinguisher. Carbon dioxide is liberated and it forces steam of effervescing liquid on to the fire. As a result, carbon dioxide surrounds the combustible substances and cuts off the supply of air. Thus, it helps to put off the fire.
- It is used in the manufacture of aerated water also known as soda water.
