Chemical Kinetics: Rate of a reaction
Chemical kinetics is the study of reaction rates, how reaction rates change under varying conditions (how fast reactants consumed, and products are formed), and what molecular events occur during the overall reaction (mechanism – pathway or series of steps for reaction).The reaction rate depends on the characteristics of the reactants and products and the conditions under which the reaction is run. By understanding how the rate of a reaction is affected by changing conditions, one can learn the details of what is happening at the molecular level (mechanism or steps required for reaction).
The reaction rate is a positive quantity that expresses how the concentration of a reactant or product changes with time.When the rate is measured by the consumption of a reactant, researchers most probably apply a opposite of positive sign to the equation to get a positive reaction flow.
Where “-”(-ve sign is placed for reactants is to keep net rate positive. Negative (-) means consumed; positive (+) means produced. Likewise, ∆R will be a negative number and the rate produced equals rate consumed. Also, [ ] means molarity (mol/L) of the constituent.
The equation above gives the average rate over the time interval, ∆t.It is defined as the average rate of change in a given quantity during a specific period.It is deduced by the slope of a line joining any two points of the time interval along the concentration- time curve.
Rates measured at the beginning of the reaction, which is dependent on the initial concentrations of reactants is called the initial rate. The instantaneous rate at time t = 0. Tangent to the curve at t = 0. The initial rate gives the best kinetic data. on such point, there is no product in the way to completely force difficulties to the data. If ∆t is short, you obtain an instantaneous rate, that is, the rate at a specific point in time.It is found by the slope of a line tangent to the curve at a point (your time in question).
Consider the gas-phase decomposition of dinitrogen pentoxide.
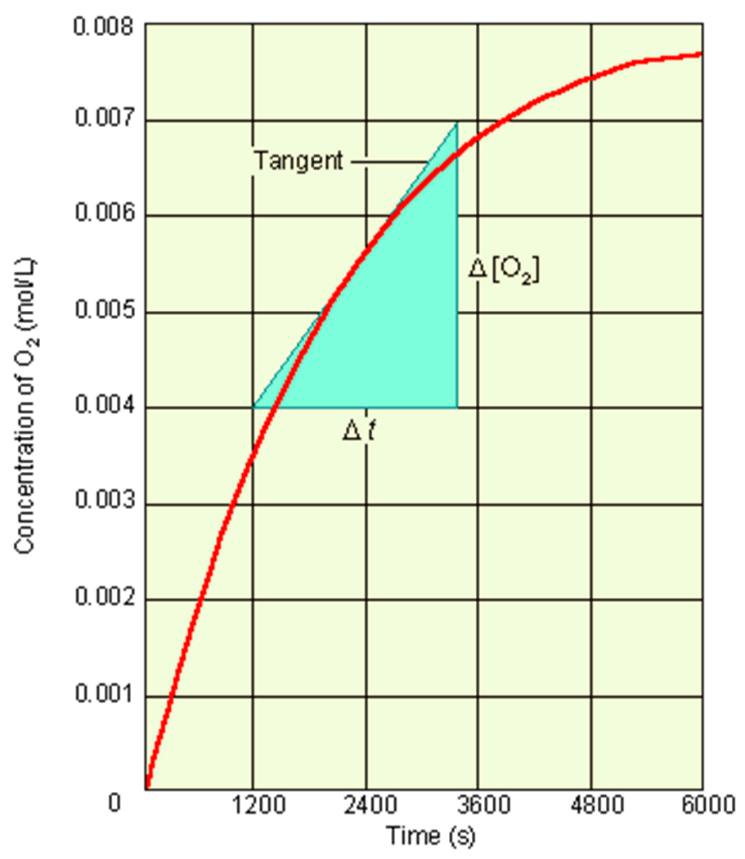
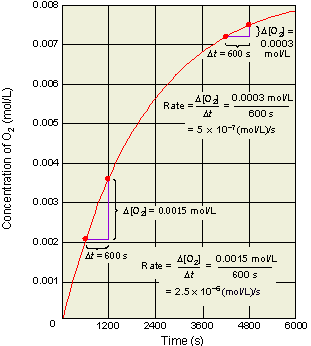
Fig The instantaneous rate of reaction.
The concentration of O2 increases over time. You obtain the instantaneous rate from the slope of the tangent at the point of the curve corresponding to that time.
When the time changes from 4200 s to 4800 s, the average rate has slowed to 5 x 10-7 mol/(L.s).
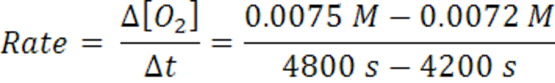
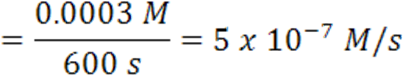
Thus, the rate of a reaction decreases as the time increases and the reactionproceeds .
When the time goes from 600 s →1200 s, the average rate is2.5 x 10-6 mol/(L.s).
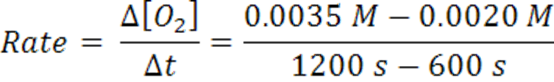
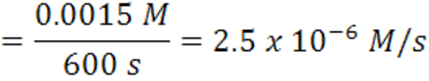
Note that the rate decreases as the reaction proceeds and it is dependent on time and concentration. It decreases as the reaction mixture runs out of reactants. Hence, concentration less for reactants, therefore,fewer collisions and slower formation of products (smaller probability for collision/reaction). Over any time, there is a certain concentration of reactants and a particular rate.Reactions occur as the result of collisions between reactant molecules; higher concentration, the greater probability of collisions, faster reaction. As reactants are consumed, their concentration drops, collisions occur less frequently, reaction rate decreases, eventually going to zero when all reactants are consumed.
