While you may be seeking to recognize the relationship between Gibbs free energy and emf of a cell, it is quite important to take a look on the basic and even the most complex elements that may be involved for the same.
What is Gibbs free energy?
Gibbs free energy could be determined as the thermodynamic potential signifying the reversible or maximum work performed by a thermodynamic system under constant pressure and temperature. The work done by electrical power within one second is described as the product of the total charge passed and emf of the cell.
Gibbs free energy equation
ΔG = ΔH – TΔS
Here,
ΔG represents Gibbs free energy
ΔH is the enthalpy change
T is temperature, and
ΔS is the change in entropy
- Energy reaction is spontaneous when ΔG < 0.
- Energy reaction tends to be non-spontaneous when ΔG > 0. And;
- The reaction will be at equilibrium when ΔG = 0.
Gibbs free energy and emf of the cell
W = nFE (cell)
Here,
W = work done,
nF = total charge passed and;
E(cell) = emf of the cell
When the charge is reversibly passed through the galvanic cell, it would be noted that the maximum amount of work is completed by the galvanic cell. The amount of reversible work done by the galvanic cell results or is responsible for the decrease in Gibbs energy within the reaction.
Δr G = −W
Δr G = −nFE(cell)
The above equation could be used also in order to calculate the standard cell potential. While the concentration of all considerable reacting species is same, E(cell) = Eo(cell) (i.e. standard cell potential and emf of a cell are equal).
⇒ Δr Go = −nFEo(cell)
The Gibbs energy of a reaction is known to be an Extensive Thermodynamic Property which indicates that its value depends on n. Thus, when two cell reactions involve varying values of n, it is observed that the values of Gibbs free energy also vary.
For example,
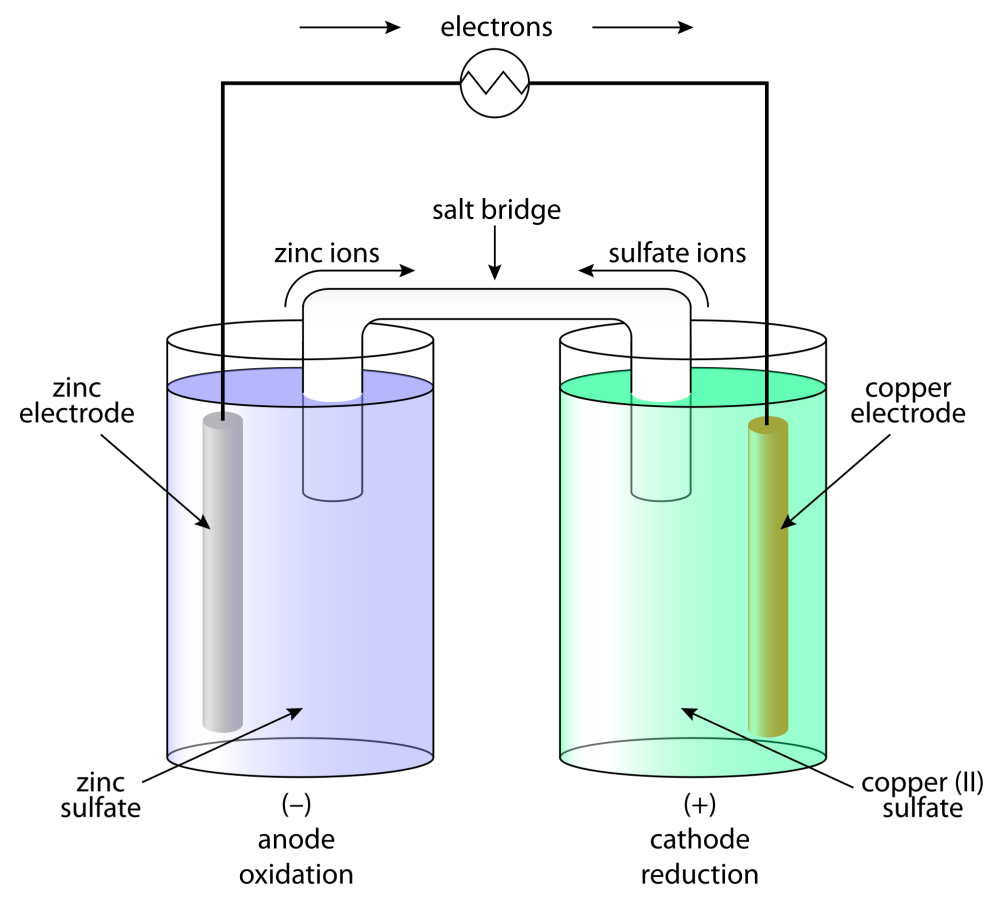
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
Δr G = −2FE(cell)
Whereas,
after the multiplication of the LHS and RHS by 2, it is observed that,
2Zn(s) + 2Cu2+(aq) → 2Zn2+(aq) +2Cu(s)
Δr G = −4FE(cell)
As per thermodynamics, Gibbs energy of a reaction could be related to a reaction quotient and when the reaction is found at equilibrium, it could be related to equilibrium constant. Considering the fact that the Gibbs energy of a reaction is dependent on emf of a cell, the equilibrium constant of a cell reaction can be related to the standard cell potential.
When at equilibrium,
Δr Go = −nFEo(cell)
ΔrGo = − RT ln K
−nFEo(cell) = − RT ln K
Eo(cell) = (RT/nF) ln K
Here,
K is equilibrium constant
R is universal gas constant
From the above-mentioned equation, we can conclude:
- When K > 1, Eo(cell)> 0, the reaction would be spontaneous and will be in favor of the formation of the product.
- When K < 1, Eo(cell) <0, the reaction is likely to be non-spontaneous and will be in favor of the formation of reactants.
The above explanation is perfect to clarify the relationship between Gibbs free energy and the emf of a cell.
