For all the aromatic compounds resonance structures can be drawn. Resonance is the way to describe the bonding, in certain ions or molecules by the combination of the various contributing structures, into a hybrid structure or resonance hybrid in the valence bond theory. Resonance has the particular value to describe the delocalized electrons, within certain polyatomic ions or the molecules, where it is not possible to express the bonding by the single Lewis structure.
Generally, resonance is a term that is referred to like the structures, which cannot be easily represented by the single electron dot structure but these are intermediate between the two or more drawn structures. The structures of the resonance are stabilizing in the molecules as they allow the electrons to increase their wavelengths and thereby their energy is lowered. This is due to the reason that the heat of formation of benzene is lower than that of predicted by the chemists as they are not accounting the resonance. The stability of other aromatic molecules is similar and it causes an overall entropic preference for the aromaticity.
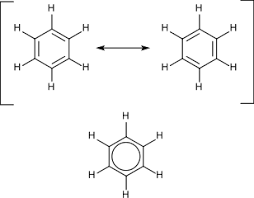
The stability of resonance has a significant role in organic chemistry and the resonant molecules, lowers the energy of formation. The resonance structures are the diagrammatic tools, which are predominantly used for the aromatic hydrocarbons for symbolizing the resonant bonds between the atoms in the molecules. Their electronic density is spread all over the molecule that is also known as delocalization of the electrons. The resonance contributors for the same molecule has the same sigma framework and the same chemical properties but pi electrons are differently contributed among the atoms.
Often resonance structures are used for the approximation of the true electronic structure as Lewis dot diagrams are not able to represent the true electronic structure of the molecule. Organic chemists frequently use resonance structures. The key elements of the resonance structures in the aromatic hydrocarbons are the following.
- Resonance occurs due to the overlapping of the orbitals. Double bonds are formed from the overlapping of 2p orbitals and are made up of pi bonds. The electrons in the pi orbitals are spread over more than the two atoms, thus they are delocalized.
- Both unshared and paired electrons can be delocalized, but all of these electrons should be conjugated in the pi system.
- The resonance structure cannot be true, if the orbitals do not overlap or if they do not mix.
- For the same molecules, the sigma framework should be the same for all the resonance structures. Resonance structures that have arbitrary separation of charges are not important. They only do minimum contribution for overall bonding position, however, in some cases like for the carbonyl group, they are important.
- Species of the aromatichydrocarbons that have the resonance structures are considered more stable than those which do not have the resonance structures. The orbital energies are lowered due to the delocalization of the electrons and their stability is imparted. There are two resonance forms of allyl cation and positive charge is delocalized over the terminal methylene groups in the hybrid structure.
