Specific Heat Capacity
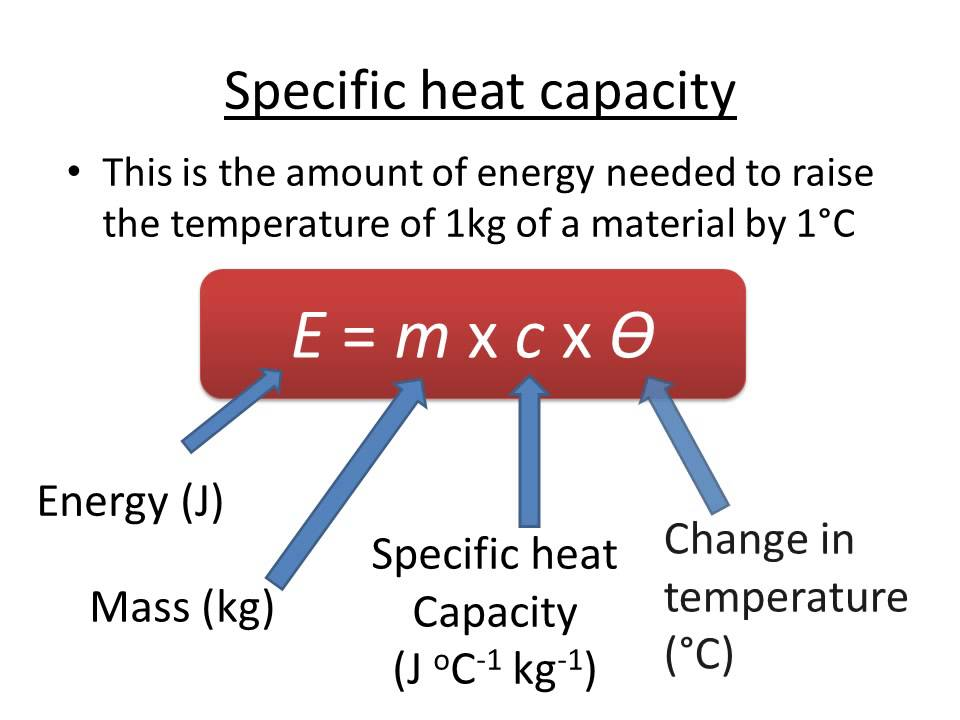
Specific heat capacity is the amount of energy required for raising the temperature of the substance per unit of the mass. The specific heat capacity of the materials is a physical property. It is also a good example of the extensive properties as its value is proportional to the size of the system being examined.
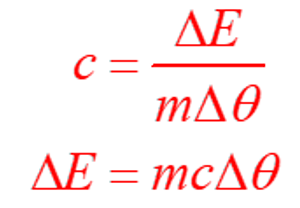

Units of Specific Heat Capacity
In the SI units, the heat capacity is defined as the amount of the heat in the joules which is required to increase the 1 gram of the substance by the I kelvin. The specific heat energy can also be expressed as J/Kg.K/. Sometimes it is also reported in the units of Calories per gram degree Celsius. Molar heat capacity and volumetric heat capacity are the related values to the specific heat capacity. The specific heat capacity of the gasses can be measured at the constant volume by enclosing the sample in the rigid container. However, for the solids and liquids, the measurement of the heat capacity can be difficult as there will be the needs of the impractical pressures for preventing the expansion that can be caused by even the smallest increase in the temperature.
Examples of Specific Heat Capacity
The specific heat capacity of the water is 4.18 J. This value is much higher than the specific heat capacity values of the other substances. Due to this reason the water is exceptionally good at the temperature regulations.
Applications of the Specific Heat Capacity
The specific heat capacity can be measured and defined for the solids, liquids, and gasses having fairly general composition and the molecular structure. These include the solutions, alloys, mixture of gasses, and heterogeneous materials, such as granite, milk, sand, and concrete, if they are considered sufficiently at a larger scale. In the cooking utensils, the material having the low specific heat is used. In this way, their bottoms can be heated more quickly. Due to this reason, the copper and aluminum polished bottoms are preferred. However, for making the handles of the cooking utensils, the material of having the high specific heat is used, which will help to sustain the heat by protecting the hands, on touching the utensils during the cooking. The insulating materials also have high specific heat. For example, woodhouse is more useful in low or high-temperature areas. Water also has a high specific heat capacity, and this is the reason that the water in the swimming pools is always cool, as compared to its outside temperature.
