The amines are basically derivatives of the ammonia. The ammonia is a compound with Nitrogen in the middle and three hydrogen atoms connected to the nitrogen. The nitrogen atom also has two valence electrons in its orbitals. The amines are generally colourless in its pure form. But, it may change colour due to oxidation with air. The amines are used on a large scale in the manufacturing of different products used in our day today lives. The amines are used in the preparation of medicines, pesticides, and dyes.
The amines are classified on the basis of the number of the alkyl or the aryl groups attached to the nitrogen group. Hence, there are the primary, the secondary and the tertiary amines. There are also the cyclic amines.
The structure of amines
Primary amines
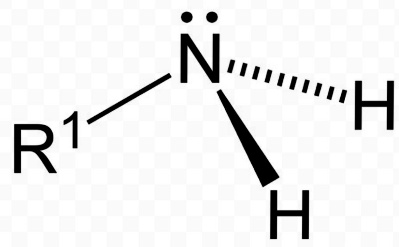
The primary amines are also known as the 1° amines. They are also famous by the name of amino. The primary amines have two hydrogen atoms attached to the nitrogen atom. The nitrogen atom of the primary amine also has two valence electron as a ligand in its orbital. This makes the compound more electron dense. The primary amines are firmed by the replacement of a hydrogen atom from the ammonia. Thus, the primary amines also act as a nucleophile in certain reactions with alkyl halides. This property is primary amines help in the further reactions for producing a higher degree amines.
The primary amines have one alkyl or an aryl group attached to the nitrogen atom. The alkyl group may consist of any saturated or unsaturated hydrocarbon with a different number of carbon atoms present in it.
Secondary amines
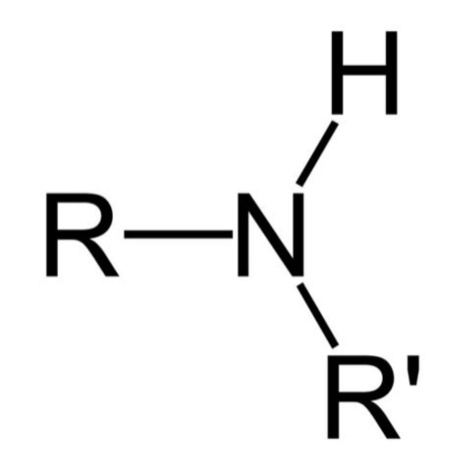
The secondary amines are also known as the 2°amines. The structure of the secondary amines consists of two hydrocarbon groups attached to the nitrogen atom. There is also a hydrogen atom bonded with a single bond to the nitrogen. The nitrogen atom of the secondary amine also consists of a ligand. The secondary amines are derived from the ammonia, by the replacement of two hydrogen atoms of the nitrogen atom with an aryl or an alkyl group respectively. The secondary amines are formed by the nucleophilic substitution of the primary amines in the alkyl halides. The secondary amines also have hydrogen, hence it also acts as a nucleophile.
Tertiary amines
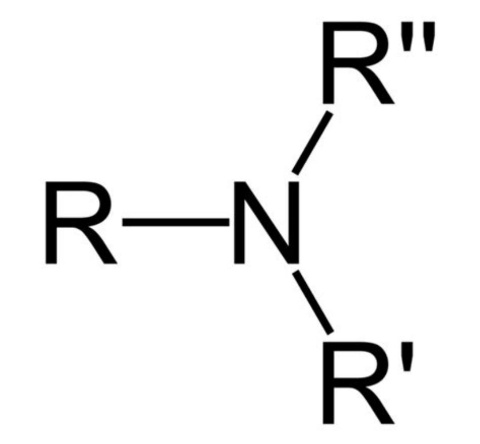
The Tertiary amines are the 3° degree amines. These amines are derived by replacement of all the hydrogen atoms from the ammonia. The tertiary amines consist of three alkyls or aryl group attached to the nitrogen atom, along with that the nitrogen atom has a ligand. The tertiary amines can be formed by the reaction of secondary amines with alkyl halides.
Quaternary amines
These are not basically amines. These are quaternary ammonia salts. They have four alkyl or aryl groups attached to the nitrogen atom.
