VB or Valence Bond theory presumes that the whole of the bonds are “localized bonds” which are formed between 2 atoms by donating an electron from each of the atoms. This is indeed a false assumption as several atoms bond with the use of delocalized electrons. As per the prediction in Molecular oxygen (O2) Valence Bond theory, there is no case of unpaired electrons.
The Valence Bond theory performs a good job of describing the quality of shapes of the covalent compounds. In general, the Molecular Orbital (MO) theory is acceptable to understand bonding. It is even more difficult when it comes to learning but helpfully predicts molecules’ actual or true properties more superior than Valence Bond theory.
Molecular Theory indeed predicts “Electron Transitions” due to the difference in the orbitals’ energy levels present in the molecule. At several situations, the theory of MO proves to be more appropriate and it is preferred due to this reason.
The Valence Bond theory details out the formation of covalent bond along with molecules’ electronic structure. The theory presumes that the electrons occupy the electron clouds or atomic orbitals of distinctive atoms inside a molecule and also, that electrons of a single atom are attracted towards the nucleus of the other atom.
The valence bond (VB) theory was explained by London and Heitler. The VB theory was majorly based on ideas of an “electronic configuration of elements”, “atomic orbital” or “electron cloud”, the hybridization of electron clouds, overlapping of electron clouds.
The overlapping of electron clouds results as the chemical bond formation. Because of the overlapping, localized electrons are found in the bond area.
Molecules’ electronic structure is being described by VB theory. In this, the nucleus of one atom gets attracted by the electrons present in another atom. So, now see the different assumptions of VB theory.
Assumptions of Valence Bond Theory
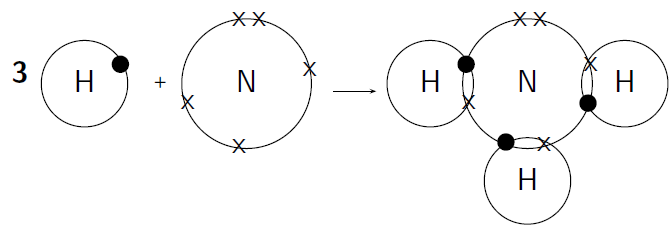
- A covalent bond is formed as a result of overlapping two 50 percent filled “valence orbital” of 2 different atoms. Overlapping gives rise to electron density which increases present between two bonded atoms. It results in advancing the stability property of the molecule.
- If atomic orbitals consist of an unpaired electron which is more than one, the formation of more than one bond takes place and valence shell electron pair can’t participate in corresponding bond formation.
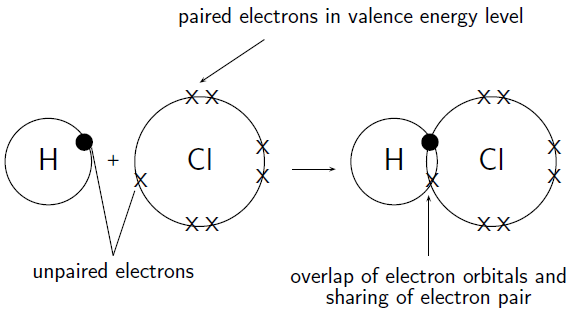
- The covalent bond possesses a directional nature. This type of bond is even parallel to the overlapping atomic orbits area.
- Depending on the overlapping pattern, covalent bonds are of 2 types: pi (Π) and sigma bond (σ). A pi bond is referred to as the formation of the covalent bond by sidewise atomic orbitals’ overlapping while the formation of the bond by atomic orbital’s overlapping alongside inter nucleus axis is called a sigma bond.
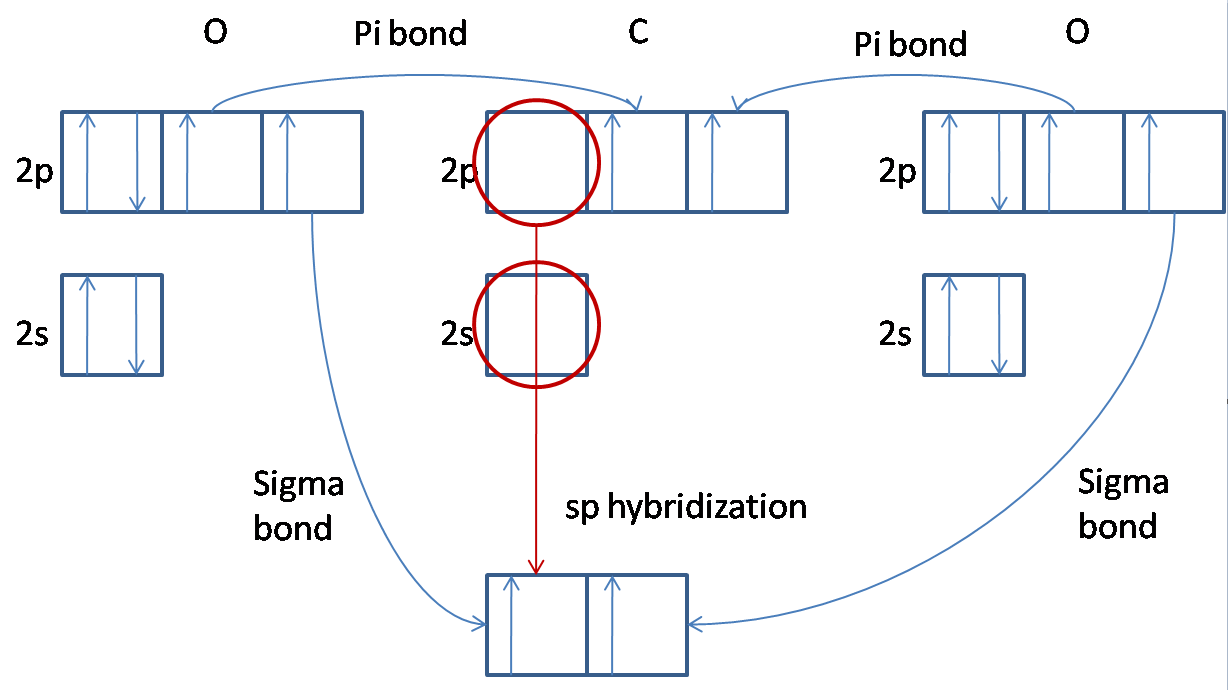
Limitations of Valence Bond Theory
The Valence Bond theory (VB) is not that perfect. It surely has its own limitations as mentioned below:
- It’s unsuccessful in explaining carbon’s tetravalency.
- This VB theory doesn’t consider discuss the energies of the electrons.
This attraction steps high and high as the atoms come closer to one another till the atoms attain a minimum or marginal distance from where electron density starts the repulsion process in between the 2 atoms. The electron density at this point which is present at the least distance in between 2 atoms is the point where the minimum potential energy is obtained, and it can be studied as what dominates the two atoms jointly in a molecular bond, or covalent bond or a chemical bond.
