The ones prevailing on the outer shell of the nucleus of an atom are called valence electrons. Valence electrons are generally helpful to provide a deeper insight into the chemical properties of any element. Irrespective of them being electropositive or electronegative, they will let you have the idea of the bond order for a specific chemical compound which determines the relevant number of bonds that could be formed using two atoms.
Since the formation of covalent bonds is dependent on how the electrons are shared in the outer shell, it is likely to clarify the bonds could be formed for elements. The interaction between different atoms and the procedure of formation of various chemical bonds, therefore, has a lot to do with valence electrons.
Valence Electrons
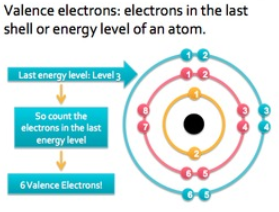
Significance of Valence Electrons
The probable number of electrons that an atom needs to gain or lose in order to obtain the nearest inert gas (noble gas) configuration is its valence. It is generally the number of electrons that are required to fill the outer shell. With respect to the electrons in the other shells, the ones in the outer shell are much more powerful. This is also the reason why they are the most considerable parts of a chemical reaction. Giving an idea about how readily, atoms could be involved in forming bonds, and the number of atoms can take part in the reaction and the number of unshared electrons; they convey several important details about the element they belong to.
Characteristics of Valance Electrons
Electrons are known to have an active participation in the reaction and chemical bond formation among atoms. They are supposed to acquire the orbits around the nucleus. The periodic table could help you know the number of valence electrons for various elements. The number of valence electrons for an atom is known to be similar to the group number that is delegated to an element. An outer shell or valence shell filled entirely with electrons means that atom is stable. Atoms generally share their electrons in a way that they can obtain an outer shell that is completely filled with electrons. Mentioned below are a few common characteristics of valence electrons:
- The valence electron for the said main group of elements prevails in the outermost or valence electron shell.
- For a transition metal, the valence electron could find its place in one of the inner shells too.
- An atom with a closed shell, completely filled with electrons is regarded to be chemically stable or inert; attaining the noble gas configuration.
- Valence electrons either release or absorb energy. This energy is known to exist as a photon.
- These electrons are also responsible for determining electrical conductivity of a specific element; considering the fact that it is a metal, metalloid or non-metal.
Determining Valence Electrons
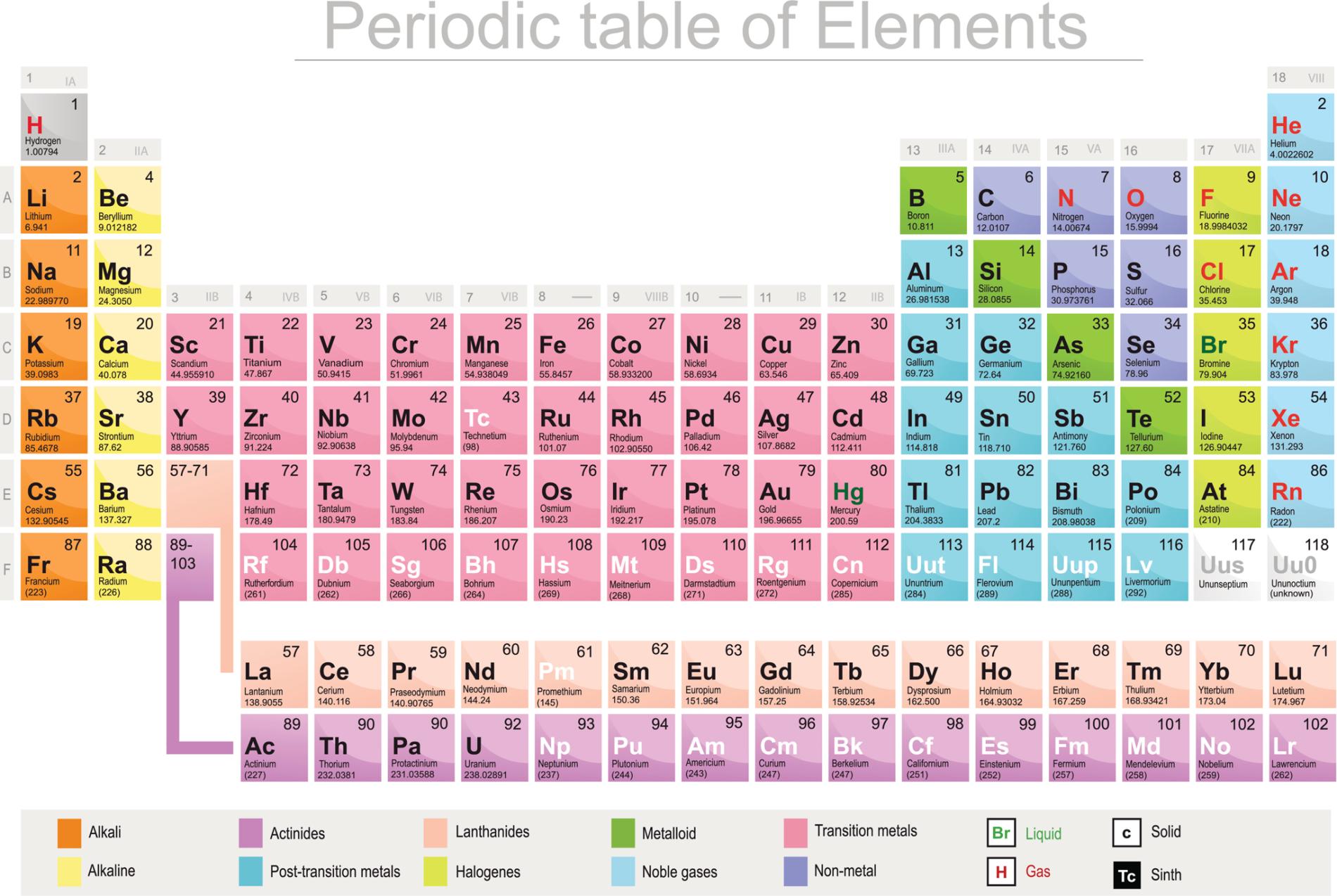
One of the most common ways to know the number of valence electrons is by referring the position of an element in the periodic table. Taking a close view across the vertical column in which the element has been grouped will let you know how many valence electrons prevail for the same. The group number on which the element had been enlisted would help in knowing the valence electrons with its electronic configuration. It is however, difficult to know the count of valence electrons for transition metals since the atomic structure for these elements is rigid.
