p-Block Elements: Coordination compounds
VBT and CFT
| Valence bond theory (VBT) | Crystal field theory (CFT) |
| Provides chemical bonding of atoms in a molecule in ionic and covalent structures. | Provides the only electronic structure of coordinate complex where the bonding is purely ionic |
| Explains the formation of a covalent bond via hybridization of atomic orbitals. | Explains the breaking of degeneracies of electron orbitals due to the electrostatic field produced by a surrounding anion or anions explanation |
| It assumes that more overlap increases the strength of an orbital. | It assumes that a central atom is surrounded by ligands that are point charge or point dipole. |
| Based on the overlapping of orbitals to form a chemical bond | It assumes that is no interaction between metal orbitals and ligand orbitals. And splitting of d orbital occurs due to ligands. It disregards covalent bonding and orbital overlap in metal complexes |
| Can show the shape but not the geometry of the molecules. The σ bonds for liner and π bonds for parallel overlap are applicable | Cannot predict the shape of the complexes |
| Indicates the type of bonding present between atoms | Indicates the spectroscopy, magnetism, and reactivity trends of d-block compounds |
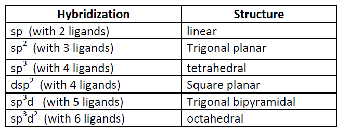
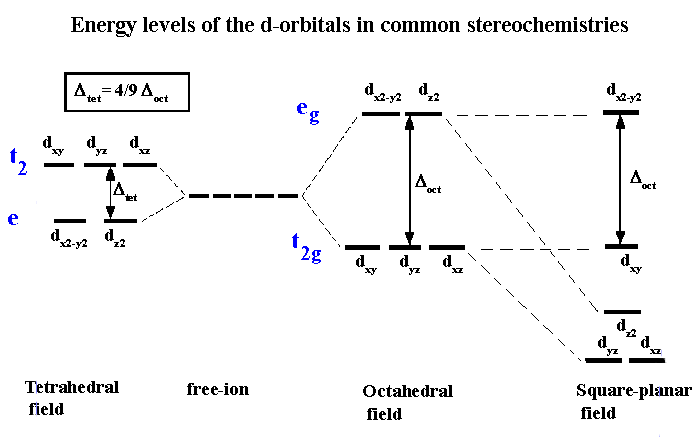
| The metal atom or ion under the influence of ligands can use (n-1)d, ns, np or ns, np, nd, nf orbital for hybridization to yield a set of equivalent orbitals of definite geometry such as octahedral, tetrahedral, square planar and so on | All the five d-orbitals are degenerate (same energy) in the isolated, gaseous metal ion. |
| It does not give a quantitative interpretation of the magnetic spin of the electrons or explain the colour exhibited by various forms of coordination compounds with the same molecular formula. | Since the CF model is an ionic model based on the electrostatic effect of the ligands on the energies of the metal ion d orbitals, it is surprising to find that anionic ligands such as Br–, F– and OH– should be weaker-field than neutral ligands such as CO, NH3 and PR3. This anomaly arises because the CFA model does not take account of covalence in metal-ligand bonding. |
| It does not make exact predictions especially for the tetrahedral and square planar structures in the case of 4-coordinate complexes. | Shows exact structural representation of tetrahedral splitting and square planar splitting |
| It does not distinguish between weak and strong ligands. | Ligand strength: (Weak) I–< F–< H2O < NH3< CN– (Strong) Metal strength: (Weak) Mn2+< Ni2+< Co2+< Fe2+< V2+< Fe3+< Cr3+< V3+< Co3+< Mn3+< Mo3+< Rh3+< Ru3+< Pd4+< Ir3+< Pt4+ (Strong) |
 |  |
