p-Block Elements: Coordination compounds
Werner’s theory
In 1893, Alfred Werner proposed that metal ions exhibit what he called primary and secondary valences. Consider, ammonia complexes of cobalt (III) for explaining these valencies.
- Primary valences denoted the oxidation number for the metal (+3 on the cobalt ion at the right).
- Secondary valences denoted the coordination number, the number of atoms directly bonded to the metal (denoted as 6 in the coordinate complex at the right).
The central metal and the ligands directly bonded in the coordination sphere of the complex. In CoCl3∙6 NH3, all six of the ligands are NH3 and the 3 chloride ions are outside the coordination sphere. In CoCl3∙5 NH3, cobalt is bonded to five NH3 groups and one chlorine while the other two chloride ions are outside the sphere.
Werner proposed putting all molecules and ions within the sphere by using brackets and the “free” anions that dissociate from the complex ion in water placed to the outside of the brackets. This approach allowed the prediction of two forms of CoCl3 ∙ 4 NH3. Likewise, the formula is written as [Co(NH3)4Cl2]Cl and one of the two forms has the two chlorines are placed next to each other while the other complex has the chlorines opposite each other.
He largely studied compounds with four or six ligands such as Octahedral and square-planar complexes. He explains multiple complexes of the same sets of ligands in diff. numbers where different numbers of ions are produced due to outer sphere dissociation. For example:
[Co(NH3)6]Cl3 [Co(NH3)5Cl]Cl2 [Co(NH3)4Cl2]Cl [Co(NH3)3Cl3]
He also explained multiple complexes with exact same formula = isomers and finally convinced Jorgenson by synthesizing the following compounds:
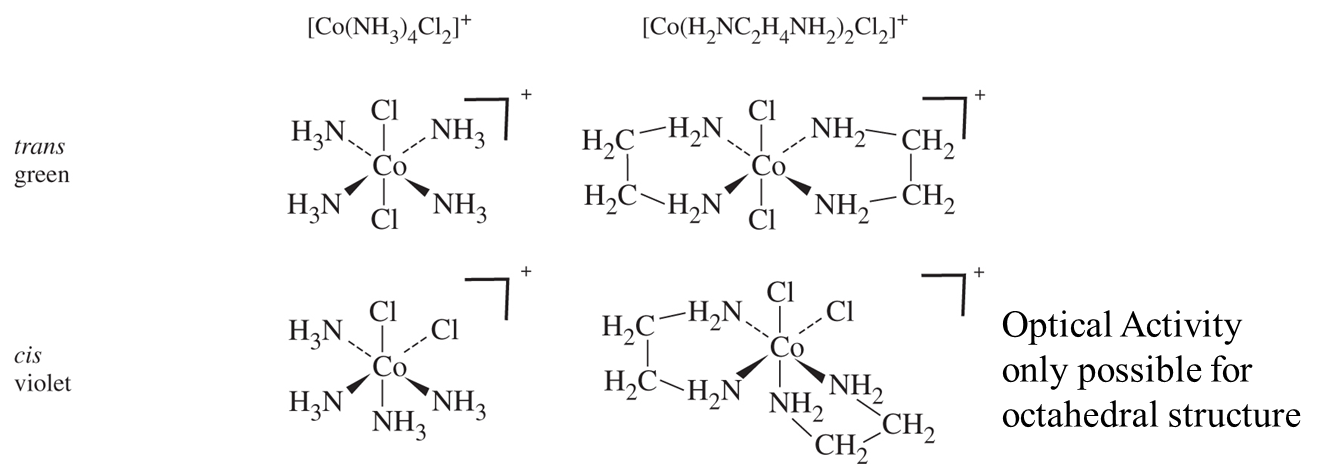
Further ideas of Werner proved correct as well. His use of “inert” or slow-reacting metal ions (Co3+, Cr3+, Pt2+) allowed successful isolation of isolates from its isomers
- Coordination Number = the number of ligands a given metal ion prefers to have bound. Most first-row transition elements prefer 6. Pt2+ prefers 4 = square planar geometry.
- CoA4B2 only has two isomers. Trigonal Prismatic and Trigonal Antiprimatic should give 3 isomers. Octahedral only has two possible isomers. Thus the structure must be Oh.
- PtA2B2 only has two isomers. It must be square planar and not tetrahedral so that it would only have 1 isomer.
- Water completes the Inner Sphere coordination in aqueous solutions:
NiCl2 + H2O [Ni(H2O)6]Cl2
For his work on Coordination Chemistry, Werner was awarded the Nobel Prize in 1913
